Atom Model Basics Explained-2025
Overview of Atomic Models
Understanding the atom is fundamental to grasping the intricacies of chemistry and physics. The journey to our current understanding of atomic structure is a story of scientific curiosity, experiments, and evolving theories. From the earliest musings to advanced quantum mechanical models, each step has paved the way for the next.
Early philosophers like Democritus pondered the existence of indivisible particles, but it wasn’t until the scientific revolution that more concrete ideas emerged. John Dalton’s early 19th-century model marked the beginning of modern atomic theory, introducing the concept of unique atoms for each element. However, his model couldn’t explain the internal structure of atoms, prompting further investigation.

As the 20th century approached, discoveries accelerated. J.J. Thomson’s identification of the electron revealed that atoms were not indivisible. His plum pudding model posited that electrons were embedded within a positively charged “soup.” This notion was quickly challenged by Ernest Rutherford’s gold foil experiment, which identified a dense central nucleus, revolutionizing the understanding of atomic structure.
Building on Rutherford’s findings, Niels Bohr introduced the idea of energy levels, where electrons orbit the nucleus in defined paths. His model explained atomic emission spectra, providing insights into atomic stability. Yet, it was limited to simpler atoms like hydrogen, indicating the need for a more comprehensive approach.
The quantum mechanical model, emerging in the mid-20th century, offered a sophisticated description of atomic behavior. Unlike previous models, it viewed electrons as existing in probability clouds rather than fixed orbits. This approach integrated the wave-particle duality of electrons, presenting a more accurate portrayal of atomic interactions.
Each model brought new insights, refining the understanding of atomic structure and behavior. The progression from Dalton’s solid spheres to the quantum mechanical model reflects the continuous advancement of scientific knowledge and technological innovation. Today, atomic theory remains a dynamic field, with ongoing research promising to unveil deeper layers of complexity within the atomic world.
Initial Theories of the Atom
In the early 19th century, John Dalton introduced the idea that elements are composed of distinct atoms. He proposed that atoms were indivisible, solid spheres, much like tiny billiard balls, and that each element had its own unique type of atom. Dalton’s model was revolutionary because it provided a way to explain why elements combine in specific ratios to form compounds, a concept rooted in the law of definite proportions.
Despite its groundbreaking nature, Dalton’s model had limitations. It couldn’t account for the internal structure of atoms or explain the intricacies of chemical reactions. This left many scientists curious and driven to probe deeper into the atom’s structure.
Prior to Dalton, the atom was merely a philosophical idea, with no experimental evidence to back it. Dalton’s work transformed the notion of atoms from abstract speculation to a concrete scientific concept, setting the stage for future breakthroughs.
Dalton’s atomic theory consisted of several key postulates: each element is made of tiny, indivisible particles called atoms; atoms of a given element are identical in mass and properties; atoms cannot be created or destroyed in chemical reactions, only rearranged; and compounds are formed when atoms of different elements combine in fixed ratios. These ideas laid the groundwork for modern chemistry by providing a framework for understanding chemical behavior and reactivity.
As science progressed, it became clear that Dalton’s model was too simplistic. The discovery of subatomic particles, such as electrons, protons, and neutrons, indicated that atoms were not indivisible. This realization prompted further exploration into the atom’s internal structure, leading to more sophisticated models that could better explain atomic behavior and the results of various experiments.
The quest to understand the atom’s true nature has been a driving force in the field of science, leading to the development of models that have increasingly revealed the complexity of atomic structure.
Thomson’s Model: The Plum Pudding Theory
J.J. Thomson’s identification of the electron marked a significant shift in the understanding of atomic structure. Unlike Dalton’s solid sphere model, Thomson’s plum pudding theory proposed that atoms consist of electrons embedded within a positively charged medium. This medium, or “soup,” was thought to spread out uniformly throughout the atom, with electrons scattered within it like plums in a pudding.
Thomson’s model emerged from his experiments with cathode rays, where he observed particles much smaller than atoms. He concluded these particles, later named electrons, were components of atoms. The discovery challenged the notion of indivisible atoms and introduced the idea that atoms have internal structure.
The plum pudding model attempted to explain how these negatively charged electrons could coexist within an atom. Thomson suggested that the positive charge in the atom was diffused evenly, balancing the negative charge of the electrons. This arrangement was believed to maintain the overall neutrality of the atom. Although the model offered a novel way to envision atomic structure, it could not account for how electrons were organized or why they did not spiral into the positive “soup.”
The plum pudding theory faced significant challenges and was soon contested by new experimental evidence. While it provided an initial framework for incorporating electrons into atomic theory, it lacked the ability to explain the results of subsequent experiments, such as those conducted by Rutherford. These limitations highlighted the need for more refined models to describe atomic behavior accurately.
Thomson’s contributions were pivotal in shifting scientific perspectives on the atom. His work laid the groundwork for future research that would eventually reveal a more complex and accurate depiction of atomic structure. Despite its shortcomings, the plum pudding model represents an important step in the ongoing journey to understand the fundamental nature of matter.
Rutherford’s Discovery: The Nuclear Atom
Rutherford’s experiments in 1911 fundamentally changed the understanding of atomic structure. By directing alpha particles at a thin sheet of gold foil, Rutherford observed that most particles passed through, but some were deflected at sharp angles. This unexpected result suggested the presence of a dense central core within the atom, which he termed the nucleus. Prior to this discovery, the prevailing model was Thomson’s plum pudding theory, which could not account for such deflections.
Rutherford concluded that the atom consists mostly of empty space, with a tiny, dense, positively charged nucleus at its center. This nucleus contains most of the atom’s mass, while the electrons orbit around it. The nuclear atom model proposed by Rutherford drastically shifted the scientific community’s view, highlighting that the atom is not a uniform mass but has a distinct internal structure.
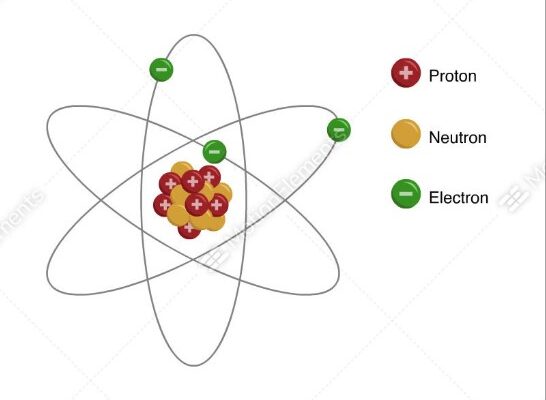
The nucleus, as identified by Rutherford, is composed of protons, which are positively charged particles. This central nucleus is small compared to the overall size of the atom, with the electrons occupying the vast, surrounding space. The discovery of the nucleus also explained why alpha particles were deflected; they were repelled by the concentrated positive charge in the nucleus.
Rutherford’s findings laid the groundwork for subsequent atomic models, including Bohr’s theory of quantized energy levels and the later development of the quantum mechanical model. His gold foil experiment demonstrated that atoms have a complex internal structure, disproving the idea that they were indivisible or homogenous. This pivotal discovery opened new avenues for understanding atomic behavior, setting the stage for the intricate models that followed.
Bohr’s Concept of the Atom
Bohr expanded upon Rutherford’s model in 1913 by introducing the concept of energy levels. He proposed that electrons orbit the nucleus in specific paths or shells, with each orbit corresponding to a distinct energy level. This idea explained atomic emission spectra, where electrons jump between energy levels, emitting or absorbing light at specific wavelengths.
Bohr’s model brought clarity to atomic stability, as it suggested that electrons remain in stable orbits without radiating energy, thus preventing them from spiraling into the nucleus. The concept of quantized energy levels marked a significant departure from previous models, which did not account for the discrete nature of atomic spectra. Bohr’s theory introduced the idea that electrons could only occupy certain allowed orbits, each associated with a specific energy state.
Despite its successes, Bohr’s model had limitations. It accurately described the hydrogen atom but struggled to explain the spectra of more complex atoms with multiple electrons. The theory couldn’t account for the intricacies of electron interactions in atoms with higher atomic numbers. Nonetheless, Bohr’s contributions provided a foundational framework for future advancements in atomic theory.
The introduction of energy levels paved the way for the development of more comprehensive models, ultimately leading to the quantum mechanical model, which addressed the shortcomings of Bohr’s theory. The concept of quantized orbits remained influential, offering valuable insights into atomic behavior and guiding subsequent scientific inquiries into the nature of matter.
The Quantum Mechanical Atom Model
The quantum mechanical model, which emerged in the mid-20th century, offers a sophisticated and nuanced understanding of atomic structure. This model shifts from earlier conceptions by describing electrons not as particles in fixed orbits, but as existing within probability clouds. These clouds represent areas where an electron is most likely to be found at any given time, based on a mathematical function called a wave function.
One of the key aspects of this model is the incorporation of the wave-particle duality of electrons, a fundamental principle of quantum mechanics. Electrons exhibit both wave-like and particle-like properties, and their behavior can be described by Schrödinger’s equation. This equation allows scientists to calculate the probability distributions of electrons around the nucleus, offering a more accurate depiction of their behavior compared to previous models.

The quantum mechanical model also introduces the concept of atomic orbitals, which are regions within the probability cloud where electrons are most likely to be found. These orbitals have distinct shapes and orientations, such as spherically shaped s-orbitals and dumbbell-shaped p-orbitals. The arrangement of electrons in these orbitals determines the chemical properties of an element and its behavior in reactions.
Additionally, the quantum mechanical model accounts for the Heisenberg Uncertainty Principle, which states that it is impossible to know both the exact position and momentum of an electron simultaneously. This principle underscores the inherent limitations in measuring atomic and subatomic particles with absolute precision.
Another significant feature of the quantum mechanical model is the Pauli Exclusion Principle, which asserts that no two electrons can occupy the same quantum state simultaneously. This principle explains the electron configurations of atoms and the periodic trends observed in the elements.
Overall, the quantum mechanical model provides a robust framework for understanding the complexities of atomic behavior. It integrates key principles of quantum theory to describe the distribution and interactions of electrons in a manner that aligns with observed experimental data.
Wrapping Up: The Progression of Atomic Models
The development of atomic models reflects the dynamic nature of scientific inquiry and the incremental steps taken to refine our understanding of the microscopic world. From the early musings of indivisible particles to the detailed quantum mechanical model, each theory has built upon its predecessor, incorporating new discoveries and addressing the limitations of earlier ideas. These advancements have not only clarified the structure of the atom but have also deepened our understanding of the fundamental principles governing matter.
Dalton’s solid sphere model introduced the concept of unique atoms for each element, laying the groundwork for modern chemistry. Thomson’s identification of the electron challenged the notion of indivisibility and proposed a new perspective on atomic structure with his plum pudding model. Rutherford’s groundbreaking gold foil experiment revealed the dense, central nucleus, revolutionizing the understanding of atomic composition. Bohr then introduced quantized energy levels, explaining atomic stability and emission spectra for simple atoms.
The quantum mechanical model has provided the most comprehensive and accurate depiction of atomic behavior to date. By viewing electrons as existing in probability clouds and incorporating wave-particle duality, this model aligns with observed experimental data and offers a nuanced understanding of electron distribution and interactions.
As we continue to investigate the complexities of atomic structure, ongoing research promises to reveal even more intricate details. Future advancements may lead to new models or refinements of existing ones, pushing the boundaries of our knowledge and potentially unlocking new technological innovations. The study of atoms remains an exciting and evolving field, with each discovery bringing us closer to a deeper understanding of the universe at its most fundamental level.